Uses of Sulphuric Acid
The chemical or molecular formula of Sulfuric Acid is H 2 SO 4Sulfuric Acid is one most important commercially used chemicals. ZIMSEC O Level Combined Science Notes.
Uses Of Sulphuric Acid Qs Study
Sulphuric acid is a colourless odourless.

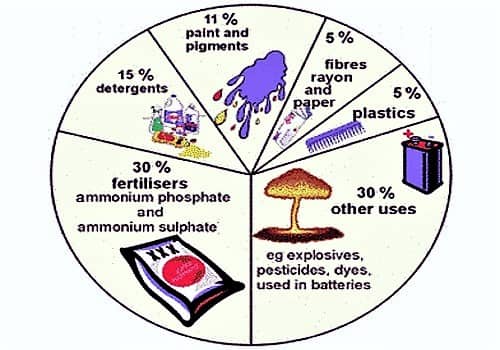
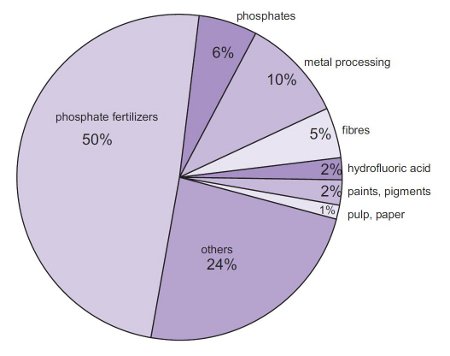
. For sulfuric acid USEPAOPP Pesticide Code. Which process is used for the preparation of Sulphuric acid. The major use of sulfuric acid is in the production of fertilizers eg superphosphate of lime and ammonium sulfate.
The chemical formula of sulphuric acid is H 2 SO 4. Sulfuric acid CAS RN. Ad Find discounts on Sulfuric acid.
Sulphuric acid contains one sulphur atom four oxygen atoms with two hydrogen atoms which are attached to two of the oxygen atoms forming the -OH group. It is highly soluble in water. Uses of sulphuric acid.
Which in turn is used as a flocculent in water purification processes. The reason that sulfuric acid household products are so common has to do with its corrosive properties. Sulfur dioxide and oxygen passed over a hot catalyst unite to form sulfur trioxide.
078001 ACTIVE products with label matches. Uses of Sulfuric Acid. The sulfuric acid used in lead-acid batteries has a pH of 05 which is the same as 335 sulphuric acid H 2 SO 4.
Sulphuric acids chemical formula is H2SO4. Most of the sulphuric acid produced at Zimphos is used to. The rate of chemical reactions is determined by.
Registered for use in the US. Its boiling point is 340 and its colourless crystals melt at 1038. Ad Browse discover thousands of brands.
This makes it an excellent choice for products such as toilet bowl cleaners and drain cleanersopeners. The H-bonding between sulfuric acid molecules shows a high boiling point and viscosity. It is also known as Mattling acid or Hydrogen Sulfate or VitriolSulphuric acid is a very strong acid and viscous liquid.
Ad Lab Scale-Up and Production Sizes. It is widely used in the manufacture of chemicals eg in making hydrochloric acid nitric acid sulfate salts synthetic detergents dyes and pigments explosives and drugs. Click Here for Sample Questions The mineral acid sulphuric acid is defined below.
It is extensively used in the production of chemicals eg in making hydrochloric acid nitric acid sulfate salts artificial detergents dyes and pigments explosives and. Uses of Sulphuric Acid H 2 SO 4 The uses for sulfuric acid are immeasurable and it is usually found in manufactured chemicals ranging from composition to explosives. Other sulfuric acid uses include additives in powdered laundry detergents hand soap dishwashing liquid and pet products.
The chemical formula of this compound is H2SO4. Sulphuric acid gives rise to phosphoric acid which produces phosphate fertilizers. It is soluble in water and is a component of acid rain.
It has a density of 184 at 15 which does not fume. Sulphuric acid is an oily liquid that is viscous in nature. It is mostly used in the production of fertilizers.
Priced Right and Ready To Ship. But approved pesticide uses may change periodically and so federal state and local authorities must be consulted for currently approved uses. Bauxite from Mutare is dissolved into.
Pure sulfuric acid is a colourless dense heavy and syrupy oily liquid. It has largely replaced the chamber or lead-chamber process. Lab Chemicals - Top Quality Solvents Reagents Acids.
Make fertilizers mostly superphoshate Some of the sulphiric acid is used to manufacture aluminum sulphate. What Is Sulphuric Acid. Search Save Online Today.
Read customer reviews find best sellers. It can oxidise both metals and non-metals with itself getting reduced to SO 2. Hot and concentrated H 2 SO 4 is a moderately strong oxidising agent.
For your reference the fundamental ideas pertaining to sulfuric acid sulfuric. Sulphuric acid contains 2 hydrogen atoms 1 sulfur atom and 4 oxygen atoms in its chemical formula. Ad Free 2-day Shipping On Millions of Items.
The reverse reaction is how to make sulfuric acid. Contact process Contact process modern industrial method of producing sulfuric acid. Sulphuric acid is also a strong dehydrating agent.
It is a very potent inorganic acid. Sulphuric acid is the most often employed acid in a variety of chemical studies. Sulfuric Acid or Sulphuric Acid is a mineral acid consisting of one Sulfur four Oxygen and two Hydrogen atoms.

Sulfuric Acid H2so4 Ppt Video Online Download

Ic 6 Uses Of Sulfuric Acid Youtube

Sulfuric Acid Structure Formula Uses Facts Britannica

Uses Of Sulphuric Acid Youtube
0 Response to "Uses of Sulphuric Acid"
Post a Comment